Health Canada’s recent SAP revision brings a new opportunity for patients and a clear responsibility for prescribers.
Health Canada’s recent decision to include psychedelic medicines in its Special Access Program (SAP) was met with a lot of fanfare. The SAP amendment brings good news for certain patients – specifically, treatment-resistant patients suffering from serious mental health conditions that impact individuals, families, and communities.
The new federal amendment has the potential to fill a critical gap for patients in need, including those suffering from depression, PTSD, and end-of-life anxiety. Many who suffer from mental health conditions don’t respond fully to current treatments, so there is a significant unmet need for safer and more effective therapies. The change to Health Canada’s SAP now allows physicians, clinics and hospitals to apply for previously restricted drugs for medical use, providing a new option for the patients who need it most.
I applaud the federal government for responding to the grave situation of the patients who aren’t responding to otherwise adequate treatment – and for recognizing the encouraging clinical data around psychedelic-assisted therapy. This SAP revision represents one small but important step on the road to greater access to psychedelic medicine.
Like most opportunities, this one comes with considerable responsibility. Failure to act responsibly could cause harm to individuals and to this evolving area of medicine. However, I believe that the community of experts in psychedelic medicine are ready and willing to support the practitioners who will be administering these therapies to patients.

What Does the SAP Revision Provide?
Health Canada’s SAP revision adds certain psychedelics, including MDMA and psilocybin, to the list of restricted substances that practitioners can request to treat patients in specific situations. Decisions will be made on a case-by-case basis, and will be reserved for serious treatment-resistant or life-threatening conditions, in instances where other therapies have failed, or are unsuitable or not available in Canada.
The recent amendment reverses regulatory changes made almost a decade ago that prohibited access to restricted drugs (including psychedelics). Historically, practitioners in Canada have been able to apply for unlicensed medications only through Health Canada’s Section 56 exemption – a fairly long and restrictive process. The SAP revision is expected to provide a much quicker review and more rapid access for approved patients.
Obviously, the SAP amendment will not bring broad access to psychedelic medicine in Canada, but ideally will help treatment-resistant patients, and serves as a clear signal that the government is acknowledging the potential of psychedelic medicine as a legitimate treatment option.
Celebrate the Progress, Continue the Push for Approval
To me, the government’s decision to include psychedelics in Canada’s SAP is a key acknowledgement that mental health conditions are being placed on the same footing as physical conditions, and frankly, that’s a shift that’s long overdue. Anyone working in mental health can see that treatment-resistant mental illness is indeed a serious or life-threatening condition, analogous to cancer that hasn’t responded to conventional treatment. But mental health disorders aren’t always viewed with that sense of urgency.
I’ve dedicated a good part of my medical career to raising awareness and advocating for changes in the treatment of mental health issues. I spent more than 30 years as a medical officer and psychiatrist in the Canadian Armed Forces, deploying twice and leading mental health programs in Afghanistan. I served as mental health advisor to the Canadian Forces surgeon general, and led initiatives with Canada and NATO as we explored innovative solutions in mental health. Achieving change in attitudes toward mental health and treatment innovation requires considerable effort and persistence.

We’ve seen modest improvement in mental health care over the years. However, I firmly believe we need to do better in this arena. Far superior advances have been made in the treatment of cancer, heart disease, and many other conditions that take an enormous toll on society and represent a significant medical and economic burden.
Yet in the field of mental health, so many patients continue to suffer without adequate or effective treatment. We must review the data while being mindful that each file or data point represents a person who is struggling. We must work to develop medicines with better results, realizing that mental health disorders affect not only patients, but their families and loved ones, their careers and communities.
During my time as the Chief of Psychiatry, I have experienced firsthand the enormous impact that trauma can have on soldiers and veterans. From mass graves in Rwanda to the battlefields of Kandahar, it’s difficult to see people who are putting their lives on the line to protect their country return home to treatments that will only work for half of them.
So the onus is on us to look for better solutions, to refuse to be satisfied with the status quo and to embrace ALL positive steps forward. In Canada, the inclusion of psychedelics in the SAP is one of those steps. That’s progress worth celebrating.
A growing body of evidence continues to demonstrate that psychedelic-assisted psychotherapies are emerging as a successful treatment option in many indications, from treatment-resistant depression to smoking and alcohol addiction to PTSD, anxiety, and OCD.
In the area of smoking cessation, Dr. Matthew Johnson and his team at Johns Hopkins are planning new studies to build on his team’s ongoing research, including the first government-funded clinical study in 50 years evaluating a psychedelic for therapeutic use. The team’s earlier study reported that 80% of participants who received psychedelic-assisted therapy remained abstinent from smoking at 6 months and 67% remained abstinent at 12 months. Those encouraging results show strong efficacy, and demonstrate clear progress.
We see positive data in other indications as well, including PTSD. MAPS is currently sponsoring MAPP2, the second of two Phase 3 trials studying MDMA-assisted therapy for PTSD. In the first Phase 3 study, 88% of participants with severe PTSD experienced a clinically-significant reduction in PTSD diagnostic scores two months after their third session of MDMA-assisted therapy, compared to 60% of placebo participants. Additionally, 67% of participants in the MDMA group (compared to 32% of participants in the placebo group) no longer met the criteria for PTSD remission two months after the sessions.
When governmental and regulatory agencies endorse the positive early results of new, transformative treatments, we can celebrate this success. And when organizations dedicate funding for continued research in our field, we applaud those decisions. We can use every bit of incremental progress as adrenaline to keep gathering evidence, and to use that evidence as our guide as we expand treatment options and promote best practices in administering them.
Setting Up Providers and Patients for Success
As Canada implements its recent change, the responsibility lies with clinicians and regulatory bodies to be very deliberate and safe in the way we use the SAP program. We must ensure that patient selection is based on science, and principles such as informed consent are followed.
I encourage doctors and patients considering these new treatment modalities to review the available research and have open, honest conversations with one another to determine if psychedelic-assisted psychotherapy is right for them. These are far from being first-line treatments and we must continue to turn to approved evidence-based treatments first.
Here’s the government’s process for requesting drugs through the SAP:
To administer psychedelic-assisted therapy under Health Canada’s SAP, healthcare professionals must fill out an application, which will be reviewed on a case-by-case basis.
The SAP considers a “healthcare professional” someone who:
- is entitled, under the laws of a province or territory, to treat patients with an unapproved prescription drug
- practices in that province or territory
- has prescribing privileges in the respective province
Practitioners who receive approval can then request products from manufacturers that meet governmental requirements.
A few examples of questions asked in the application:
“What specifically about this drug makes it the best choice for your patient(s)?”
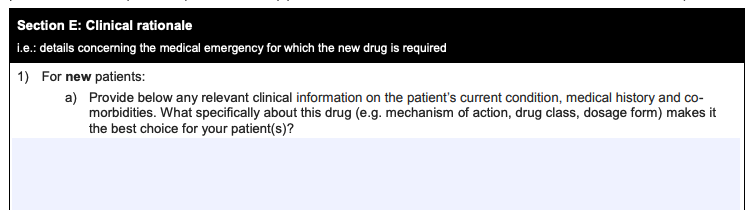
“Specify all treatments tried and/or failed…”

A request to provide references/evidence:
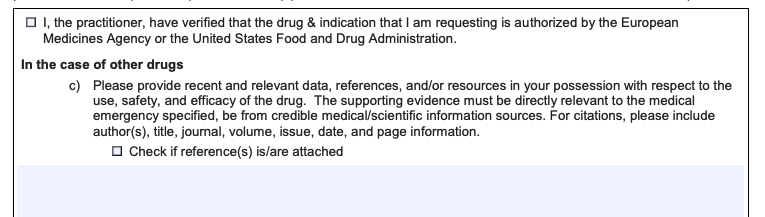
A question for a request for a repeat patient:

The final section:
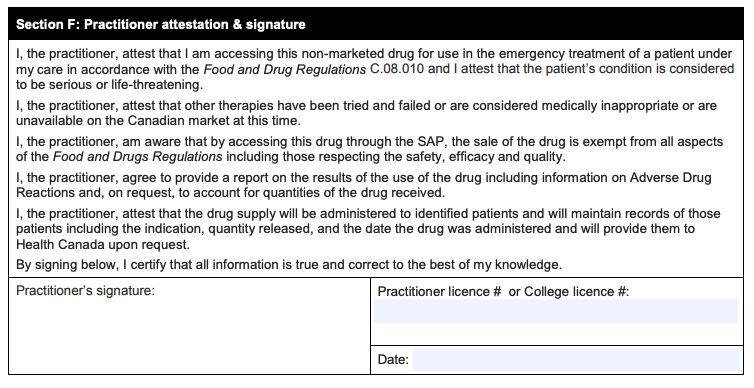
How progressive or cautious will Health Canada be in reviewing and approving requests? That remains to be seen. But as a physician, my advice is clear: The practitioners who seek permission to use these medicines should ensure that they have the necessary training, competence, and confidence to provide these treatments safely and successfully.
The innovators in our field are scientists, doctors, and advisors offering extensive experience with psychedelic compounds, as well as mental health and addiction disorders. We must step up and support physicians who want to prescribe these treatments, but who might not have experience implementing psychedelic-assisted psychotherapy. We can provide evidence-based research, education on proper protocols, and access to experienced psychedelic integration specialists to answer questions every step of the way.
My message is simple: Let’s do this right. Let’s do this safely.

The End Goal: Regulatory Approval and Integration into Clinical Practice
The SAP should not be considered an alternative to integrating psychedelic-assisted therapy into existing medical practices. Rather, it provides help for those who qualify for use in exceptional circumstances under the SAP guidelines. It’s a step forward, but it’s not a solution.
Psilocybin and MDMA-based therapies are successful with specific indications and patient profiles. We need to continue gathering data to demonstrate safety and efficacy through clinical trials targeting specific indications. That’s the path to obtain regulatory approval of psychedelics with therapy protocols. Psychedelics must undergo the same rigor as any other medication vying for approval from regulatory bodies. We need to continue the work that will lead to an environment of safe, regulated access to psychedelic therapy in a medical setting. That takes patience, but will pay off in the long run.
Ultimately, the millions of patients afflicted with serious mental illness will benefit most when they have access to more advanced, more effective therapies than those on the market today. We truly see success when medical communities view psychedelic medicine as an accepted and adopted form of treatment within our existing healthcare infrastructure.